Standards and Certification Requirements for Medical Protective Clothing
According to the Announcement No. 53 of 2020 of the General Administration of Customs of China, from April 10th, the export of medical materials under 11 categories and 19 types of customs commodity numbers is subject to export commodity inspection. Customs laboratories will test the export of medical materials in accordance with the following standards: 1) If the importing country (region) has quality and safety standards, the testing will be based on the importing country’s (region) quality and safety standards. 2) If the importing country (region) has no quality and safety standards, the testing will be based on Chinese quality and safety standards. This article will introduce the respective standards and certification requirements for medical protective clothing in China, the United States, and the European Union.
Standard Overview
The national standard for medical protective clothing in China is GB 19082-2009 “Technical Requirements for Medical Disposable Protective Clothing”. This standard is a mandatory standard for disposable, medical non-woven protective clothing. It does not provide for reusable protective clothing.
The standard for medical protective clothing in the United States is NFPA 1999, which is suitable for evaluating the barrier performance of sanitary protective clothing.
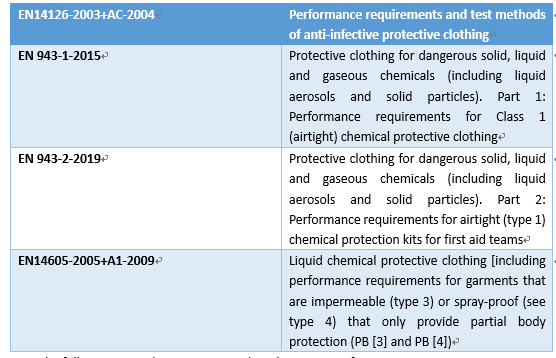
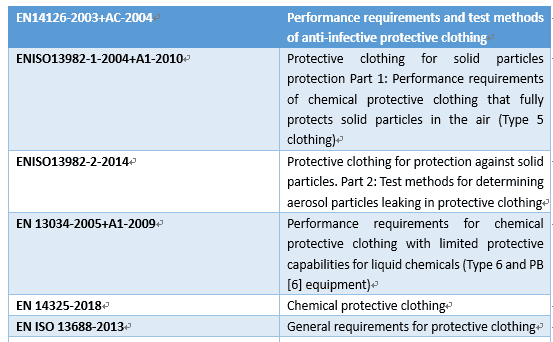
The EU has promulgated EN14126-2003 performance requirements and test methods for protective clothing and anti-virus protective clothing. The main focus of this standard is the resistance of protective clothing to the penetration of blood, body fluids, and viruses.
1. China–Standards and Certification Requirements for Medical Protective Clothing
China usually divides medical devices into three categories based on risk.
- First – medical devices with little or no risks are filed with the drug regulatory department of the prefectural city bureau.
- Second – the medium-risk medical devices are registered and approved by the provincial drug regulatory department.
- Third – high-risk medical devices are directly organized and approved by the National Bureau.
Medical protective clothing belongs to the second category of medical devices.
2. United States–Standards and Certification Requirements for Medical Protective Clothing
The US Food and Drug Administration (FDA) divides medical device products into three levels for management according to risk levels. Level 1 refers to ordinary products, level 2 refers to products that are guaranteed to be safe and effective through special control, and level 3 refers to products that support human life and prevent damage to human health but have potentially unreasonable risks.
American medical protective clothing includes two types: non-surgical protective clothing and surgical protective clothing. Non-surgical protective clothing is a Class I medical device, which is exempt from registration before going to market and can be registered directly by the institution. Surgical protective clothing belongs to Class II medical devices, which requires pre-market registration, that is, you need to apply for FDA 510K.
| Category | Product | Judgement Standard |
| Class I | Non-Medical protective clothing | 1. The label reads “Protective clothing”, not “Medical protective clothing.”
2. The label does not describe the product as a surgical gown. 3. If the product has a statement about barrier protection, it can only be declared as a low level of barrier protection. |
| Class II | Medical protective clothing | 1. The label reads, “Medical protective clothing”.
2. The label content describes the product as protective clothing for surgery. 3. The product declares that it is a medium or high level of a barrier protection that can be used for the aseptic operation. |
The procedure for registration of the Type II products before a listing is as follows:
(1) Product testing (performance testing, chemical testing)
(2) Prepare 510K files
(3) Submit to FDA for review
(4) FDA issued 510K approval
(5) Complete factory registration and machine listing
(6) Product export
3. European Union–Standards and Certification Requirements for Medical Protective Clothing
In the EU Economic Area market, it is mandatory that commodities related to safety, health, environmental protection and consumer protection (such as electronics, machinery, medical devices, toys, personal protective equipment, communications, pressure equipment, etc.) have to carry CE mark, otherwise, it cannot enter the EU market for circulation.
Protective clothing belongs to Personal Protective Equipment(PPE), and the European Union’s personal protective equipment safety directive is 89/686 / EEC. According to the provisions of the PPE Directive, PPE products are divided into three categories according to the complexity and protection level of product design. Personal protective equipment sold in the European market must comply with applicable safety requirements and standards.
To obtain CE certification, you need to perform the following steps:
(1) Apply and submit the model
(2) Submit supplier certificate and test report
(3) Evaluation and certification
(4) PPE type certificate
(5) Declaration of conformity
Comparative analysis of standard indicators
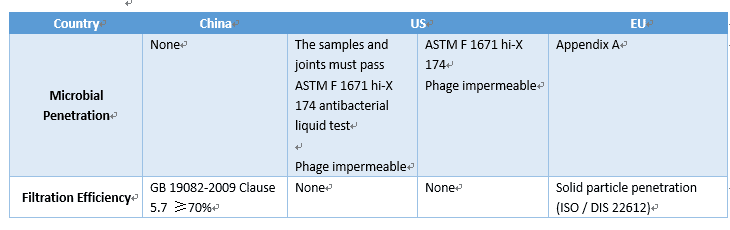
The “protective performance” of medical protective clothing is the most important performance requirement, which mainly includes the requirements of the liquid barrier, microbial barrier, and barrier to particulate matter. China first promulgated the National Compulsory Standard for “Technical Requirements for Medical Disposable Protective Clothing” in 2003 for the first time and revised it to GB19082-2009 “Technical Requirements for Medical Disposable Protective Clothing” in 2009 with relatively rich performance indicators. At present, the most common international standards for medical protective clothing are the National Institute of Occupational Safety and Health (NIOSH) standard of the United States and the EN standard of the European Union. The following is a simple comparison of the protection performance in the standards of China, the United States, and Europe for medical protective clothing.
Indicator Analysis
(1) Disposable non-woven materials/fabric are basically used for medical protective clothing internationally, but there are certain differences in the focus of protective clothing indicators, product performance requirements, and testing methods in various countries. Due to different testing methods, the key project indicators cannot be simply compared based on the data.
(2) In GB19082-2009 “Technical Requirements for Medical Disposable Protective Clothing,” the filtration efficiency of key indicators is not mentioned in other national standards. However, the standard does not involve some key indicators such as microbial penetration, anti-pollutant penetration, and other test items. Both the US and EU standards have impermeable requirements.
(3) Regarding the standards and certification requirements for medical protective clothing in terms of comfort, the Chinese, American, and European standards have different focuses. The Chinese standards have requirements for moisture permeability, while the American standards focus on the requirements for thermal performance.